Abstract
Objective Idiopathic multicentric Castleman's disease (iMCD) is a rare lymphoproliferative disorder driven by proinflammatory hypercytokinemia. Patient perspective of the burden and associated symptoms of iMCD on their daily life have not been previously studied. We developed a bespoke international online survey to investigate, characterize, and map the symptoms and associated burden on daily life experienced by patients with various subtypes of iMCD. Statistical analysis was conducted to explore the psychometric properties of the survey.
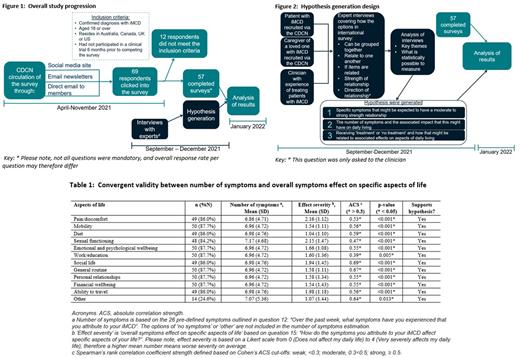
Methods A survey to elicit the burden of disease-related symptoms and their effects on daily life from a patient perspective was developed using information from clinical practice and published literature. Eligible patients were >18 years old having physician-confirmed diagnosis of iMCD. The survey was distributed within iMCD communities in Australia, Canada, the UK, and the US through the Castleman Disease Collaborative Network (CDCN).
Hypotheses were generated based on expert opinion (one clinician, one patient, and one caregiver) in one-to-one interviews to understand whether specific questions and response options within the patient questionnaire could be: 1. grouped together as the same; 2. related to one another; or 3. if items were related, the expected strength of the relationship and the potential direction of relationship. Spearman's rank absolute correlation strength and Cohen's-d standardized effect size methods, alongside estimating relevant p-values, were used to explore these relationships. The overall study programme is depicted in Figure 1, with details of the study design captured in Figure 2.
Results Overall, 57 patients with iMCD completed the survey between April-November 2021. Patients experienced a mean number of 7 symptoms a week (range: 0 to 22 symptoms), highlighting the burden iMCD on patient.
To explore the robustness of these results, three a priori hypothesis sets were generated as a result of the expert interviews: 1. how specific symptoms relate to each other; 2. number of symptoms and their relationship with aspects of daily life; and 3. receiving treatment or not, and the overall relationship with aspects of daily life.
9 symptom pairs were identified for exploration, however as the number of symptoms experienced by patients with iMCD was so high and heterogeneous, in reality only 2 of the pairs were identified to be statistically significant due to small response numbers for other pairings. There was a moderate positive relationship between tiredness and physical weakness, and a weak positive relationship between tiredness and dizziness.
There was a strong correlation between having a higher number of symptoms and greater adverse association with daily life, as depicted in Table 1.
On reflection, it was realised that cross-sectional data as is the case in this survey was not appropriate for exploring links between treatment or not, and associated aspects of daily life as we have no indication of the baseline for the patients. The collecting of longitudinal data in such future research would support such analysis and increase the likelihood of making meaningful observations. A longitudinal approach would further add value to these results be gathering information on how iMCD progresses from a patient perspective.
Conclusion To our knowledge this is the first study to characterise symptom burden of iMCD and its impact on daily living. The exploratory psychometric testing provides a level of confidence in the construct validity of the survey such that the results can hopefully be used to ultimately develop symptom burden score that can help assessment of disease severity, treatment decisions and evaluating responses in daily practice and clinical research. The exploratory testing also highlighted the challenges of working with naturally small samples sizes and the importance of recognising the limitations of what can be done with a data set given the data collection design.
Disclosures
Mukherjee:Eusa Pharma: Consultancy, Other: Advisor or review panel participant; Teaching and Speaking; Jazz Pharmaceuticals: Other: Principal investigator for Investigator Initiated Trials (the Institution gets the funding), Research Funding; BioPharm: Consultancy; Partnership for Health Analytic Research, LLC: Honoraria; Blueprint Medicines: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant, Research Funding; Aplastic Anemia and MDS International Foundation: Honoraria; Celgene/Acceleron: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant, Research Funding; McGraw Hill Hematology Oncology Board Review: Honoraria, Other: Advisor or review panel participant. Franklin:Eusa Pharma: Consultancy. Shupo:Eusa Pharma: Current Employment. Mason:Eusa Pharma: Consultancy. Wayi-Wayi:Eusa Pharma: Current Employment. Zibelnik:Eusa Pharma: Current Employment. Repasky:Eusa Pharma: Consultancy. Brazier:Eusa Pharma: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal